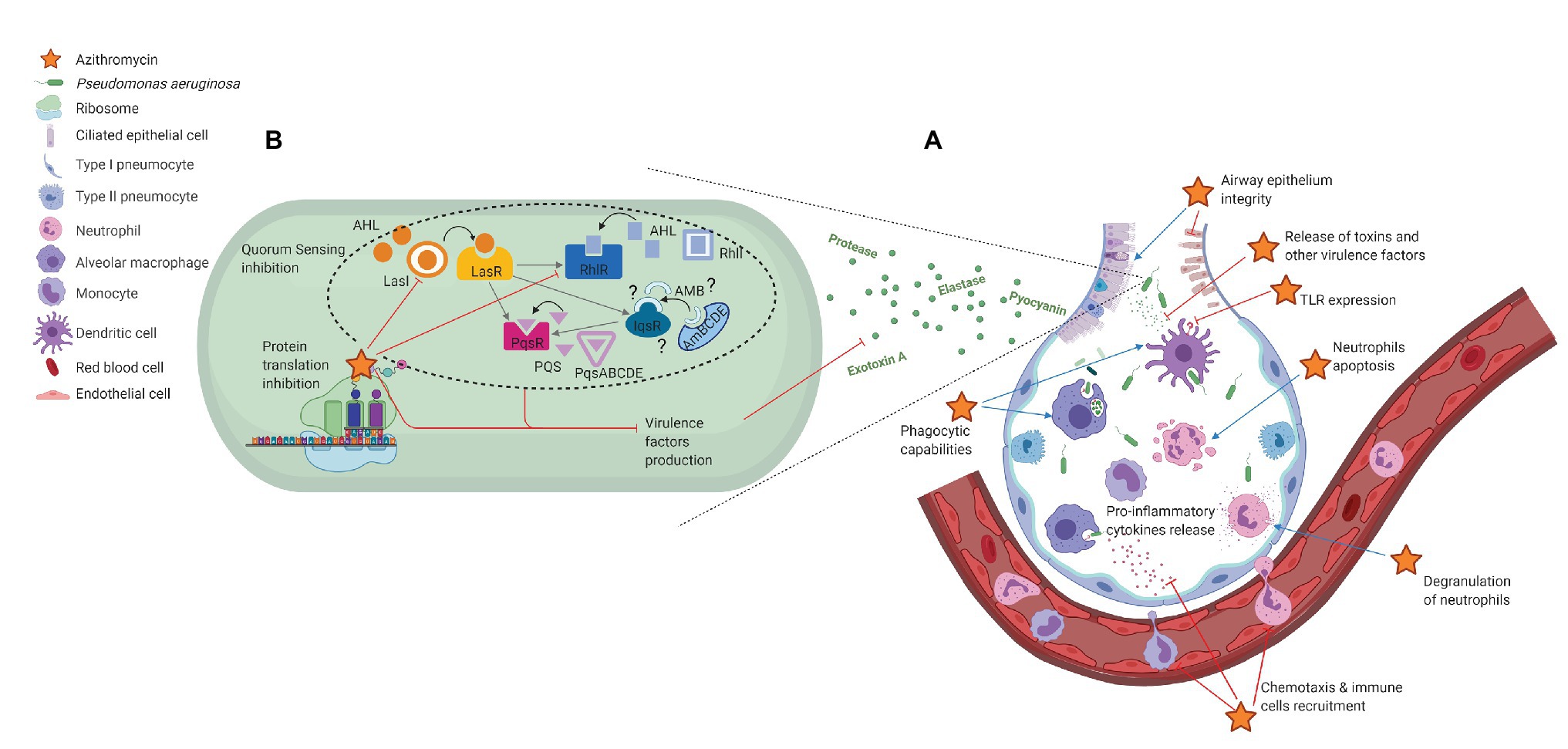
Azithromycin’s activity against Pseudomonas aeruginosa is limited. Consider alternative antibiotics, such as aminoglycosides (gentamicin, tobramycin), fluoroquinolones (ciprofloxacin, levofloxacin), or carbapenems (imipenem, meropenem), as first-line choices for treating Pseudomonas infections. These agents demonstrate superior efficacy and broader coverage.
While in vitro studies may show minimal inhibitory concentrations (MICs) for some Pseudomonas strains, clinical data consistently demonstrates poor azithromycin efficacy against this pathogen. This is largely due to Pseudomonas‘ intrinsic resistance mechanisms, notably its ability to produce efflux pumps and express aminoglycoside-modifying enzymes.
Specific recommendations: Always perform susceptibility testing to guide antibiotic selection. Relying solely on azithromycin for Pseudomonas infections risks treatment failure and potential adverse outcomes. Combination therapy, involving agents with synergistic activity against Pseudomonas, may be considered in severe infections, but should be guided by antimicrobial stewardship guidelines and local resistance patterns.
Note: This information is for educational purposes only and does not constitute medical advice. Always consult with a qualified healthcare professional for diagnosis and treatment of infections.
- Azithromycin Coverage for Pseudomonas: A Detailed Overview
- Activity of Azithromycin Against Pseudomonas aeruginosa In Vitro
- Factors Influencing Azithromycin Activity
- Clinical Implications of Azithromycin’s Limited Activity Against Pseudomonas
- Impact on Treatment Success
- Appropriate Antimicrobial Strategies
- Guiding Treatment Decisions
- Alternative Treatment Options for Pseudomonas aeruginosa Infections
- Future Directions in Combating Pseudomonas Resistance
Azithromycin Coverage for Pseudomonas: A Detailed Overview
Azithromycin demonstrates minimal activity against Pseudomonas aeruginosa. Therefore, it’s generally not considered a suitable treatment option for infections caused by this bacterium.
While in vitro studies may show some minimal inhibitory concentration (MIC) values, clinical experience consistently reveals poor efficacy. This lack of efficacy stems from azithromycin’s mechanism of action and the inherent resistance mechanisms frequently displayed by Pseudomonas.
- Mechanism of Action Limitations: Azithromycin targets bacterial protein synthesis. Pseudomonas aeruginosa often possesses mechanisms that circumvent this, leading to treatment failure.
- Resistance Mechanisms: Pseudomonas commonly exhibits efflux pumps and mutations impacting target sites, contributing to azithromycin resistance.
- Clinical Data: Clinical trials and observational studies uniformly fail to support the use of azithromycin as a monotherapy or adjunctive therapy against Pseudomonas infections.
Consequently, clinicians should avoid using azithromycin for treating Pseudomonas infections. Alternative antibiotics with proven efficacy against Pseudomonas aeruginosa, such as aminoglycosides, carbapenems, fluoroquinolones, and β-lactams, should be selected based on antibiotic susceptibility testing and clinical context.
- Always conduct appropriate microbiological testing to identify the causative pathogen and determine its antibiotic susceptibility profile.
- Select antibiotics based on susceptibility results and local antibiograms.
- Consider factors such as patient history, clinical presentation, and potential drug interactions when making treatment decisions.
Using azithromycin against Pseudomonas risks treatment failure and potentially promotes the development of further antibiotic resistance. Prioritize evidence-based antimicrobial stewardship practices to optimize patient outcomes and preserve the effectiveness of existing antibiotics.
Activity of Azithromycin Against Pseudomonas aeruginosa In Vitro
Azithromycin demonstrates limited activity against Pseudomonas aeruginosa in vitro. Minimum inhibitory concentrations (MICs) generally exceed 32 µg/mL for most isolates. This relatively high MIC suggests azithromycin is unlikely to achieve therapeutic concentrations in the setting of P. aeruginosa infection.
Factors Influencing Azithromycin Activity
Several factors contribute to azithromycin’s poor in vitro performance against P. aeruginosa. These include the organism’s inherent resistance mechanisms, such as efflux pumps and mutations in ribosomal binding sites. Furthermore, azithromycin’s intracellular penetration may be inadequate to reach therapeutic levels within P. aeruginosa biofilms, which frequently occur in infections.
Studies consistently show a wide range of MIC values, highlighting the significant variability in susceptibility among different P. aeruginosa strains. Therefore, relying solely on in vitro data for treatment decisions involving P. aeruginosa infections is not recommended. Alternative, more potent antibiotics are usually preferred.
Clinical Implications of Azithromycin’s Limited Activity Against Pseudomonas
Azithromycin’s weak activity against Pseudomonas aeruginosa necessitates careful consideration in treatment strategies. Avoid using azithromycin as monotherapy for Pseudomonas infections; it’s simply not potent enough. This limitation directly impacts treatment outcomes.
Impact on Treatment Success
Studies consistently show significantly lower eradication rates of Pseudomonas infections when azithromycin is used alone. This reduced efficacy translates to prolonged illness, increased risk of complications, and potentially higher mortality rates in severe cases. Therefore, always opt for combination therapy using anti-pseudomonal agents with proven efficacy.
Appropriate Antimicrobial Strategies
For treating Pseudomonas infections, clinicians should prioritize antibiotics with demonstrated activity against this bacterium. Effective options include aminoglycosides (e.g., gentamicin, tobramycin), fluoroquinolones (e.g., ciprofloxacin, levofloxacin), carbapenems (e.g., imipenem, meropenem), and β-lactam/β-lactamase inhibitor combinations (e.g., piperacillin/tazobactam).
| Antibiotic Class | Example Drugs | Activity Against Pseudomonas aeruginosa |
|---|---|---|
| Aminoglycosides | Gentamicin, Tobramycin | High |
| Fluoroquinolones | Ciprofloxacin, Levofloxacin | High (but resistance is increasing) |
| Carbapenems | Imipenem, Meropenem | High (but resistance is a growing concern) |
| β-lactam/β-lactamase inhibitor | Piperacillin/Tazobactam | High |
| Macrolides (Azithromycin) | Azithromycin | Low |
Guiding Treatment Decisions
Accurate identification of the infecting organism via culture and susceptibility testing is paramount. This informs tailored antibiotic choices, ensuring optimal treatment efficacy and minimizing the risk of treatment failure associated with insufficient antimicrobial activity. Always prioritize susceptibility testing to guide treatment decisions.
Alternative Treatment Options for Pseudomonas aeruginosa Infections
If azithromycin isn’t suitable, several other antibiotics effectively combat Pseudomonas aeruginosa. Piperacillin-tazobactam often provides a strong response. This combination targets a broad spectrum of bacteria, including many resistant strains. Consider cefepime, a fourth-generation cephalosporin, as a viable alternative; its efficacy against Pseudomonas is well-documented. For severe infections, imipenem-cilastatin, a carbapenem antibiotic, offers powerful activity.
Aminoglycosides, such as amikacin or gentamicin, frequently feature in treatment regimens, often combined with other antibiotics for synergistic effects. However, remember to carefully monitor for nephrotoxicity. Ciprofloxacin and levofloxacin, fluoroquinolones, are also options, but resistance is a growing concern; always check local antibiograms before prescribing.
Beyond antibiotics, consider adjunctive therapies. Debridement of infected tissue is crucial to remove the bacterial load in cases of localized infection. For cystic fibrosis patients, improved respiratory management, such as airway clearance techniques and inhaled medications, significantly impacts treatment success. Precise antibiotic selection depends on the infection site and the patient’s specific circumstances; consult current guidelines and local antibiograms for informed decisions.
Always prioritize susceptibility testing to guide antibiotic selection and optimize treatment efficacy. Monitoring the patient’s response is vital for adjusting therapy as needed.
Future Directions in Combating Pseudomonas Resistance
Invest in developing novel anti-pseudomonal agents targeting alternative pathways. Specifically, focus on inhibitors of efflux pumps and molecules disrupting quorum sensing.
Explore combination therapies. Simultaneous administration of azithromycin with other antibiotics, such as aminoglycosides or carbapenems, warrants further investigation, focusing on synergistic effects and reduced toxicity profiles. Clinical trials should rigorously assess efficacy and safety in diverse patient populations.
Prioritize phage therapy. Bacteriophages, viruses that infect and kill bacteria, offer a promising alternative. Research should concentrate on identifying phages with broad activity against multi-drug resistant Pseudomonas strains and developing effective delivery methods.
Enhance infection control measures. Strict adherence to hand hygiene protocols, appropriate antibiotic stewardship, and environmental decontamination strategies significantly reduce transmission and the spread of resistant strains. Data-driven strategies for optimizing these measures are needed.
Promote research into immunotherapies. Active and passive immunization strategies, harnessing the power of the host immune system, represent a novel approach. Studies focusing on specific Pseudomonas antigens and their efficacy in clinical settings are required.
Fund large-scale genomic surveillance. Tracking the emergence and spread of resistant strains through genomic sequencing allows for rapid identification of resistance mechanisms and informs the development of targeted interventions.
Develop point-of-care diagnostic tests. Rapid, accurate identification of Pseudomonas species and their resistance profiles enable timely administration of appropriate therapies. Investment in diagnostic technologies is a crucial part of combating resistance.